
Updated COVID boosters targeting Omicron start rolling out
Federal health officials say thousands of updated booster shots are already being shipped around the country.
Watch CBS News

Federal health officials say thousands of updated booster shots are already being shipped around the country.
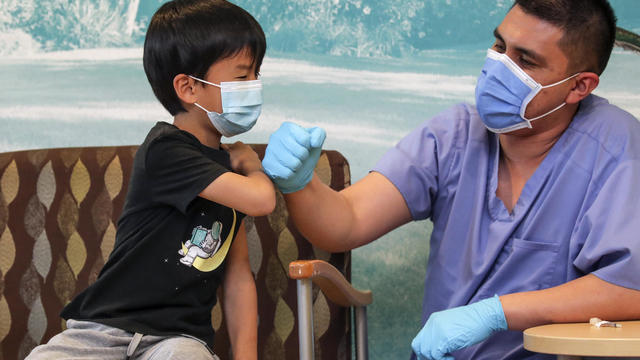
A Kaiser poll taken earlier this spring found fewer than one in five parents would get their children under 5 vaccinated right away.

The FDA's panel of vaccine advisers voted unanimously that the benefits of Pfizer's and Moderna's shots outweigh the risks in young children.
The company says it'll give U.S. regulators the data soon. Moderna is already seeking U.S. clearance to vaccinate kids 5 and under.

Citing a "robust response" of antibodies against Omicron in their vaccine trials, the companies said they were planning to submit a request "in the coming days."

Parents will have to wait a little longer for COVID-19 for their toddlers after Pfizer on Friday postponed its Food and Drug Administration application for kids under 5.
The FDA is delaying a decision on COVID-19 shots for children under 5 years of age.

Pfizer has begun a study comparing its original COVID-19 vaccine with doses specially tweaked to match the hugely contagious Omicron variant.

Antiviral pills that can significantly reduce COVID-19 hospitalizations and even deaths - but the supply of the Pfizer and Merck COVID-19 pills across the country - and here in our area - is still very limited.

"These new oral antivirals add new tools to our toolbox to keep people with COVID-19 out of the hospital," said IDPH Director Dr. Ngozi Ezike.

The CDC also recommended that kids ages 5 to 11 with moderately or severely weakened immune systems receive an additional dose 28 days after their second Pfizer shot.

The company says early results from its lab tests shows its antiviral will likely remain effective against all variants of concern, including Omicron.

The Food and Drug Administration has greenlighted a request from Pfizer and BioNTech to allow Americans as young as 16 to get a booster shot of their COVID-19 vaccine, the agency announced on Thursday, clearing a key hurdle before that age group can receive the third shot.

Pfizer is seeking authorization for its experimental COVID-19 pill after reporting it cut hospitalization and death by 90%, AP reports.

Pfizer says its COVID-19 vaccine is more than 90% effective at protecting kids from infection, ahead of public review, AP reports.

Pfizer officially submitted a request to the FDA for emergency authorization of their COVID-19 vaccine for children 5 to 11 years old.
Many people who have received Pfizer's COVID-19 vaccine are now eligible for booster shots, after the CDC gave final approval to third doses for three groups.
Pfizer says its COVID-19 vaccine works for kids ages 5 to 11. It is welcome news for many, but it also triggers a lot of questions. CBS News Chief Medical Correspondent Dr. Jon LaPook joins us with some answers on that, and on other issues surrounding the state of the pandemic.

"I'm really proud of them. Seeing them kind of courageously take this step and to do so really enthusiastically has just been a remarkable experience, not just as a pediatrician, but certainly as a dad."

Pfizer said Monday its COVID-19 vaccine works for children ages 5 to 11 and that it will seek U.S. authorization for this age group soon.
"We got our staff ready. We have the patient names ready to contact them for the booster. We are just waiting on the approval and rollout."
Full approval for the Pfizer-BioNTech COVID-19 vaccine came from the U.S. Food and Drug Administration on Monday. It came more than eight months after the FDA gave the drug authorization for emergency use.
The Food and Drug Administration announced Monday that it had granted full approval to Pfizer and BioNTech for their COVID-19 vaccine.
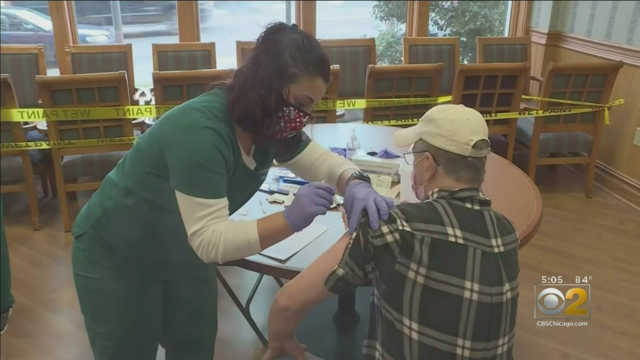
A third shot in the arm of COVID-19 vaccine? That is what Moderna suggested Thursday for this fall as cases rise, but some doctors are not on board with the idea at this time.
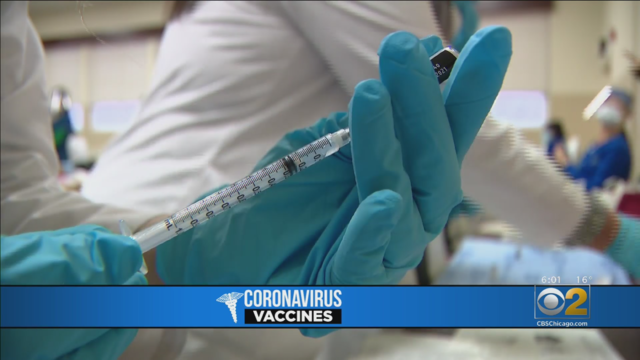
The City of Chicago is hoping to get more teens and young adults vaccinated By bringing the vaccine to local barbershops.

Breaking News, First Alert Weather, Exclusive Investigations & Community Journalism

Omar Zegar, 37, of Orland Park, was charged with one felony count of aggravated unlawful use of a weapon with a revoked FOID card.

Jennifer was charged with two counts of aggravated battery to a police officer, two counts of aggravated battery on a public way, and aggravated assault of a police officer. Sheba was also charged with two counts of aggravated battery to a police officer in addition to aggravated battery on a public way causing bodily harm and resisting a peace officer causing injury.
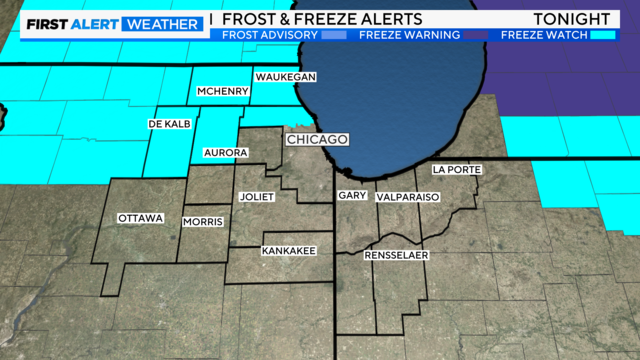
One more dry day on Thursday until a storm complex heads our way for the weekend.

The lawsuit claims workers were not given the 60 days' notice of mass layoffs required by state and federal law.

Bears president Kevin Warren said the new stadium would include replacing Soldier Field with additional parks and public sports fields, while preserving the stadium's iconic colonnades, which were built as a war memorial for American veterans.

Jurors in former President Donald Trump's trial in New York heard testimony from a former media executive about his efforts to bury negative stories about Trump before the 2016 presidential election.

Regulators prohibit new noncompetes, which impede millions of U.S. workers from getting a better job.

The Senate passed the foreign aid package, which includes a provision that could lead to a ban on TikTok, after months of disagreement in Congress.

Police arrived at the scene sooner than if they had waited for the first 911 call.

Employees at dozens of now-closed Foxtrot Market and Dom's Kitchen stores, now jobless, wonder what comes next for them as their paychecks will soon end.

The family didn't get their cut of the estate sale, not hearing back from the salesman until CBS 2 got involved.

The Better Business Bureau warns that anyone buying an event ticket should watch out for fake ticket scams.

Financial records show Paul Croft and J.D. Frost raised about $30 million for a hydrogen plant that was supposed to be, at one point, in Indiana. It never existed, an attorney says.

Anyone with these sausages in their refrigerators should throw them away or return them to the store.
The city's measles dashboard said a total of 63 measles cases have been confirmed in Chicago this year, with one new case this week.
The department said anyone who visited the Sam's Club at 9400 S. Western Ave. in Evergreen Park one day last week may have been exposed to someone with measles.

Health officials are warning consumers not to consume Infinite Herbs basil sold at some Trader Joe's and Dierberg's stores after 12 people were sickened.

Officials said there was no known link between this case and the recent outbreak of measles at a migrant shelter in Chicago.

Health department officials said the case does not appear to be linked to new migrant arrival shelters in Chicago, and the source of infection is unknown.

Employees at dozens of now-closed Foxtrot Market and Dom's Kitchen stores, now jobless, wonder what comes next for them as their paychecks will soon end.

The company announced Tuesday that it received a stalking horse bid to purchase its operating assets.

Dozens of typewriters clutter the shelves of the 24-year-old business owner's workshop inside his parents' home in Downers Grove.

The closure affects both Dom's locations in Chicago, and all 33 Foxtrot stores in Chicago, Texas, and the Washington D.C. area.

Café Selmarie, 4721 N. Lincoln Ave., first announced its plans to close in September. Owner Birgit Kobayashi is set to retire.

The singer was found deceased at her home, a representative said.

Anticipation was growing at a fever pitch before Taylor Swift's latest album, "The Tortured Poets Department," dropped at midnight EDT. But it turned out it's actually a double album.

Guitar legend Dickey Betts, who co-founded the Allman Brothers Band and wrote their biggest hit, "Ramblin' Man," has died.

Colbert lived in Chicago for 11 years, performing and "cutting his comedy teeth" at the legendary Second City and attending Northwestern University. His show will broadcast from Chicago's storied Auditorium Theater Aug. 19-22 on CBS.

The mural was unveiled on the side of the building at the southeast corner the Chicago Medical Society building at Grand Avenue Dearborn Street.

CBS 2 is partnering with local schools to fill a trolley with donations in support of United Way's April Food Day.

CBS 2 is partnering with local schools to fill a trolley with donations in support of United Way's April Food Day.

CBS 2 Meteorologist Mary Kay Kleist looks at the wet extended forecast.

The school announced the hybrid model for classes will extend to final exams. A pro-Palestinian encampment has disrupted campus life, and students have requested a remote learning option.

The bill also includes a provision forcing TikTok’s Chinese owner Byte Dance to sell the social media site or be banned in the U.S.

Bears president Kevin Warren said the new stadium would include replacing Soldier Field with additional parks and public sports fields, while preserving the stadium's iconic colonnades, which were built as a war memorial for American veterans.

The lawsuit claims workers were not given the 60 days' notice of mass layoffs required by state and federal law.

The civil rights lawsuit filed by Reed's mother accuses police of using "brutally violent, militarized policing tactics" during a traffic stop on March 21.

Crime stats of those who were carjacked, robbed, shot, or killed give an overwhelming sense of a society unmoored. But the spectacle of crime can evoke fear, often overshadowing those who are truly affected: the victims.

Omar Zegar, 37, of Orland Park, was charged with one felony count of aggravated unlawful use of a weapon with a revoked FOID card.

Police arrived at the scene sooner than if they had waited for the first 911 call.

Anthony Robinson's attorney said his previous defense lawyer failed to present evidence that showed it was physically impossible for him to be the shooter.

The village board is working on a resolution to hire Lightfoot, a former federal prosecutor before her lone term as Chicago's mayor, to investigate claims Henyard has been misusing public funds.

DCFS Director Heidi Mueller was asked Thursday why some kids in the system are being held in psychiatric hospitals longer than medically necessary.

CBS 2 discovered the woman, Whitley Temple, was hired by the City of Chicago while awaiting trial.

Bears president Kevin Warren said the new stadium would include replacing Soldier Field with additional parks and public sports fields, while preserving the stadium's iconic colonnades, which were built as a war memorial for American veterans.

The Sox joined Cincinnati in 2022, Detroit in 2003, the St. Louis Browns in 1936 and the Cleveland Spiders in 1899 as teams to start 3-20.

Cody Bellinger also hit a two-run homer in the first off J.P. France.

Right-hander Kyle Hendricks and left-hander Drew Smyly were placed on the 15-day injured list by the Chicago Cubs before a series opener against the Houston Astros.

It'll be a quick three-week turnaround for Carodoso from winning the national title game at South Carolina to her first WNBA training camp.

Police said the thieves used different types of force to make off with large quantities of cigarettes and bottles of alcohol in each incident.

School officials said police arrested the gunman and recovered a gun.

One of the men sprayed him in the face with pepper spray, while the other had a knife in his hand, police said. Both men beat until he fell to the ground, police said.

The task force isn't only responding to thefts on retail strips such as the Magnificent Mile or making arrests. They are working directly with businesses.

Two boys were shot as a large group of teens flooded the streets in the South Loop near Roosevelt and Canal on March 2.